Revolutionizing Batteries With Earth’s Most Abundant Metal


Oregon State University’s latest study introduces iron as a viable, cost-effective cathode material for lithium-ion batteries, potentially reducing reliance on costly metals like cobalt and nickel while enhancing battery safety and sustainability. Credit: SciTechDaily
New research introduces an iron-based cathode for lithium-ion batteries, offering lower costs and higher safety compared to traditional materials.
A collaborative initiative co-led by Oregon State University chemistry researcher Xiulei “David” Ji introduces iron as a viable and sustainable cathode material for lithium-ion batteries, potentially replacing costly materials like cobalt and nickel. This innovation promises higher energy density, significantly lower costs, and enhanced safety. Iron’s abundance assures a steady supply, making this development a crucial step towards more sustainable battery technology.
The research, detailed in a recent publication in Science Advances, is significant for several reasons. Ji explains, “We’ve transformed the reactivity of iron metal, the cheapest metal commodity. Our electrode can offer a higher energy density than the state-of-the-art cathode materials in electric vehicles. And since we use iron, whose cost can be less than a dollar per kilogram – a small fraction of nickel and cobalt, which are indispensable in current high-energy lithium-ion batteries – the cost of our batteries is potentially much lower.”
Economic and Environmental Benefits of Iron-Based Cathodes
Currently, the cathode accounts for half the cost of producing a lithium-ion battery cell. Iron-based cathodes could not only reduce costs but also enhance battery safety and sustainability. With the increasing demand for lithium-ion batteries to power electric vehicles, the global supply of nickel and cobalt is becoming stretched, with anticipated shortages threatening future production.
In addition, those elements’ energy density is already being extended to its ceiling level. If pushed further, oxygen released during charging could cause batteries to ignite. Additionally, cobalt is toxic, meaning it can contaminate ecosystems and water sources if it leaches out of landfills.
Put it all together, Ji said, and it’s easy to understand the global quest for new, more sustainable battery chemistries.
A collaboration co-led by Oregon State University chemistry researcher David Ji is hoping to spark a green battery revolution by showing that iron instead of cobalt and nickel can be used as a cathode material in lithium-ion batteries. Credit: Xiulei “David” Ji, Oregon State University
Understanding Battery Components and Function
A battery stores power in the form of chemical energy and through reactions converts it to the electrical energy needed to power vehicles, laptops, and other devices and machines. There are multiple types of batteries, but most of them work the same basic way and contain the same basic components.
A battery consists of two electrodes – the anode and cathode, typically made of different materials – as well as a separator and electrolyte, a chemical medium that allows for the flow of electrical charge. During battery discharge, electrons flow from the anode into an external circuit and then collect at the cathode.
In a lithium-ion battery, as its name suggests, a charge is carried via lithium ions as they move through the electrolyte from the anode to the cathode during discharge, and back again during recharging.
“Our iron-based cathode will not be limited by a shortage of resources,” said Ji, explaining that iron, in addition to being the most common element on Earth as measured by mass, is the fourth-most abundant element in the Earth’s crust. “We will not run out of iron till the sun turns into a red giant.”
Innovative Chemical Design Enhances Iron’s Reactivity
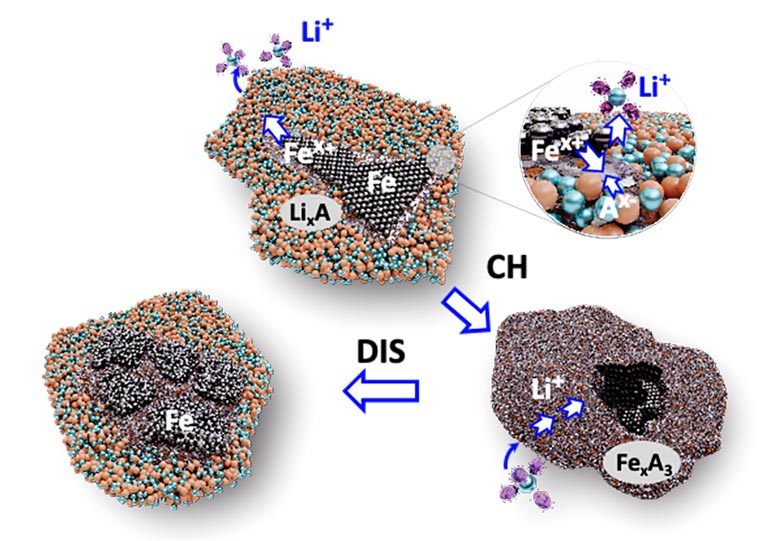
Ji and collaborators from multiple universities and national laboratories increased the reactivity of iron in their cathode by designing a chemical environment based on a blend of fluorine and phosphate anions – ions that are negatively charged.
The blend, thoroughly mixed as a solid solution, allows for the reversible conversion – meaning the battery can be recharged – of a fine mixture of iron powder, lithium fluoride, and lithium phosphate into iron salts.
“We’ve demonstrated that the materials design with anions can break the ceiling of energy density for batteries that are more sustainable and cost less,” Ji said. “We’re not using some more expensive salt in conjunction with iron – just those the battery industry has been using and then iron powder. To put this new cathode in applications, one needs to change nothing else – no new anodes, no new production lines, no new design of the battery. We are just replacing one thing, the cathode.”
Future Prospects and Environmental Impact
Storage efficiency still needs to be improved, Ji said. Right now, not all of the electricity put into the battery during charging is available for use upon discharge. When those improvements are made, and Ji expects they will be, the result will be a battery that works much better than the ones currently in use while costing less and being greener.
“If there is investment in this technology, it shouldn’t take long for it to be commercially available,” Ji said. “We need the visionaries of the industry to allocate resources to this emerging field. The world can have a cathode industry based on a metal that’s almost free compared to cobalt and nickel. And while you have to work really hard to recycle cobalt and nickel, you don’t even have to recycle iron – it just turns into rust if you let it go.”
Reference: “Unlocking iron metal as a cathode for sustainable Li-ion batteries by an anion solid solution” by Mingliang Yu, Jing Wang, Ming Lei, Min Soo Jung, Zengqing Zhuo, Yufei Yang, Xueli Zheng, Sean Sandstrom, Chunsheng Wang, Wanli Yang, De-en Jiang, Tongchao Liu and Xiulei Ji, 23 May 2024, Science Advances.
DOI: 10.1126/sciadv.adn4441
The Basic Energy Sciences program of the U.S. Department of Energy funded this research, which was co-led by Tongchao Liu of Argonne National Laboratory and also included Oregon State’s Mingliang Yu, Min Soo Jung, and Sean Sandstrom. Scientists from Vanderbilt University, Stanford University, the University of Maryland, Lawrence Berkeley National Laboratory, and the SLAC National Accelerator Laboratory contributed as well.


