The Next Big Thing in Weight Loss?


Research led by Dr. Ki-young Shin on using neuromodulation technology for appetite suppression in metabolic syndrome shows promising clinical results. The technology aims to reduce side effects compared to traditional treatments and may soon enter further research and commercial phases.
Metabolic syndrome is a complex of multiple metabolic abnormalities, including obesity, high blood pressure, and high triglycerides, often caused by poor diet and lack of exercise. According to the World Health Organization (WHO), one in eight people worldwide is overweight, making obesity treatment one of the most prominent markets currently. Although there are various types of obesity treatments including drug injections and pharmaceuticals, these chemical treatments often come with potential side effects when taken over for a long period.

Innovative Treatments: Electrical Stimulation
Now, a team of researchers led by Dr. Ki-young Shin of the Human Care Electro-Medical Device Research Center, Electro-Medical Equipment Research Division of KERI has proposed a novel approach: suppressing appetite by stimulating the cerebral cortex electrically through the scalp.
The electrical stimulation method is officially known as transcranial random noise stimulation (tRNS). After extensive research, the team discovered that non-invasive electrical stimulation of the dorsolateral prefrontal cortex—a portion of the frontal lobe that controls execution function, planning, flexibility, and abstract thinking—using tRNS technology could lead to appetite suppression.
Clinical Trials and Technological Development
Three key technologies are required for such studies.
- A technology that can accurately deliver the right electrical stimulation to the specific area of interest
- An electrode technology that can penetrate the space between the hairs and make contact with the scalp
- A monitoring technology that can confirm that the electrical stimulation has been delivered to the target point and has triggered a change in brain activity
All of these are currently under development by Dr. Shin’s team and the team possesses an advanced level of technologies.
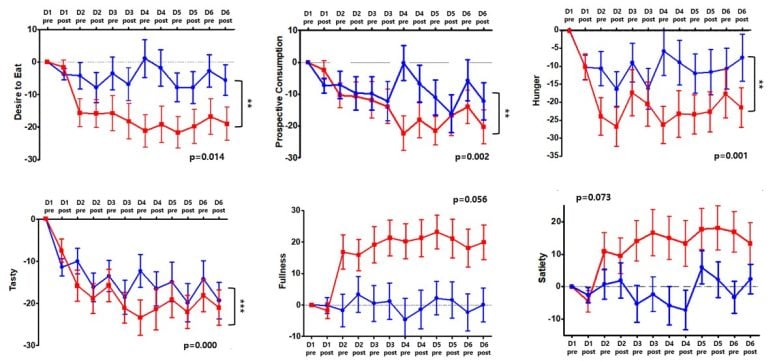
KERI conducted a clinical trial with Professor Hyung-jin Choi’s team at Seoul National University Hospital to demonstrate the clinical utility of tRNS stimulation using commercially available electrical stimulators. The goal of the clinical trial was to prove that tRNS stimulation effectively reduces appetite. The trial included 60 female volunteers, 30 in the tRNS group and 30 in the active sham group. The trial consisted of six sessions of electrical stimulation with two to three days of interval for two weeks. The electrical stimulation utilized a barely perceptible current of 2 mA for 20 minutes per session.

Implications and Future Directions
The results showed that the tRNS treatment group exhibited a reduction in appetite, willingness to eat, and hunger compared to the placebo group. The clinical trial also showed that tRNS can help treat emotional eating, meaning that the tendency to eat to process or relieve emotions such as stress, depression, anxiety, and joy was significantly reduced. As the trial was conducted only for two weeks, the long-term weight loss effect was not confirmed but participants reported significant appetite suppression.
Dr. Shin said, “Although the technology is not yet complete and needs further research and verification, if this electrostimulation treatment equipment with far fewer side effects than existing obesity treatments is commercialized and can be used at home instead of in hospitals, it will provide an easy and simple method for daily appetite suppression management.”
He added, “Especially when people are under stress or difficulty, many people eat food due to emotional hunger, and if digital healthcare technology that combines electrostimulation treatment and exercise therapy is introduced, it will enhance weight loss effects and help individuals manage their health more effectively.”
The research team is scheduled to complete the first phase of the project (2022-2024) this year and aims to validate the developed technology academically and clinically through follow-up research, including the second phase of the project, and promote technology transfer to companies.
Source link



