Scientists Reveal Molecular Secrets of Alzheimer’s Disease for the First Time
Scientists have achieved a groundbreaking discovery in Alzheimer’s research by mapping the molecular structures within a human brain using cryo-electron tomography, focusing on key proteins β-amyloid and tau associated with dementia. This study, conducted at the University of Leeds and published in Nature, provides insights into how these proteins disrupt brain functions and could pave the way for new therapeutic approaches for neurological diseases.
Breakthrough Alzheimer’s research reveals the detailed molecular structure of key brain proteins, offering new insights for potential targeted therapies.
Scientists researching Alzheimer’s disease have, for the first time, uncovered the structure of molecules within a human brain. The findings, published in Nature, detail how researchers utilized cryo-electron tomography, assisted by fluorescence microscopy, to delve into the depths of a brain donated by an Alzheimer’s patient.
This gave 3-dimensional maps in which they could observe proteins, the molecular building blocks of life a million times smaller than a grain of rice, within the brain. The study zoomed in on two proteins that cause dementia– ‘β-amyloid’, a protein that forms microscopic sticky plaques, and ‘tau’ – another protein that in Alzheimer’s disease forms abnormal filaments that grow inside cells and spread throughout the brain.
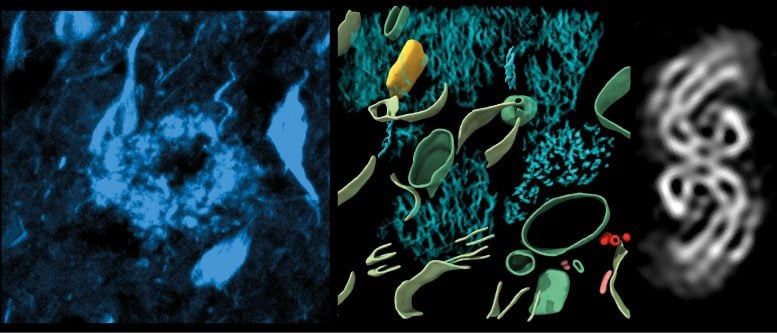
Left, fluorescence image of amyloid in cryo-preserved post-mortem human brain. Middle, 3-dimensional molecular architecture of β-amyloid plaque. Right, in-tissue structure of tau filaments within post-mortem brain. Credit: University of Leeds
This study revealed the molecular structure of tau in tissue, how amyloids are arranged, and new molecular structures entangled within this pathology in the brain. Dementia is the leading cause of death in the UK, with Alzheimer’s its most common form.
Impact of Molecular Structures on Alzheimer’s
In Alzheimer’s disease, both β-amyloid plaques and abnormal tau filaments are thought to disrupt cellular communication, which leads to symptoms such as memory loss and confusion, and cell death.
Dr Rene Frank, lead author and Associate Professor in the University of Leeds’s School of Biology, said: “This first glimpse of the structure of molecules inside the human brain offers further clues to what happens to proteins in Alzheimer’s disease but also sets out an experimental approach that can be applied to better understand a broad range of other devastating neurological diseases.”
Over the past 70 years, a vast catalog of molecular structures has been accumulated by several thousand scientists across the globe, each working with proteins in isolation in a test tube. However, it has long been known that most functions in biology are the consequence of an orchestra of many different proteins.
This study carried out at the University of Leeds in collaboration with scientists at Amsterdam UMC, Zeiss Microscopy, and the University of Cambridge, is part of new efforts by structural biologists to study proteins directly within cells and tissues, their native environment – and how proteins work together and affect one another, particularly in human cells and tissues ravaged by disease. In the longer term it is hoped that observing this interplay of proteins within tissues will accelerate identifying new targets for next-generation mechanism-based therapeutics and diagnostics.
Reference: “CryoET of β-amyloid and tau within postmortem Alzheimer’s disease brain” by Madeleine A. G. Gilbert, Nayab Fatima, Joshua Jenkins, Thomas J. O’Sullivan, Andreas Schertel, Yehuda Halfon, Martin Wilkinson, Tjado H. J. Morrema, Mirjam Geibel, Randy J. Read, Neil A. Ranson, Sheena E. Radford, Jeroen J. M. Hoozemans and René A. W. Frank, 10 July 2024, Nature.
DOI: 10.1038/s41586-024-07680-x