Scientists Capture a Never-Before-Seen Elemental Bond

A team of scientists led by the U.S. Department of Energy’s (DOE) Oak Ridge National Laboratory (ORNL) recently made an unprecedented observation of how promethium, a rare element, forms chemical bonds in aqueous solutions.
This groundbreaking discovery was made using the Beamline for Materials Measurement (BMM), a beamline funded and operated by the National Institute of Standards and Technology, at the National Synchrotron Light Source II, a DOE Office of Science user facility at DOE’s Brookhaven National Laboratory.
Applications and Mysteries of Promethium
Although promethium is rare, it has several interesting applications, including manufacturing specialized glow-in-the-dark paint, radiation therapy, and long-lasting atomic batteries for pacemakers, and spacecraft. Due to its high instability, there is still a lot about this radioactive metal that remains unknown. Understanding its complex chemistry could pave the way for even more unique uses and fascinating follow-up studies.
Observing the Lanthanide Contraction
Promethium is what is known as a “lanthanide” or “rare-earth metal.” This metal is one of 15 elements that occupy the lower portion of the periodic table and carry the atomic numbers 57 through 71. While these metals look and feel fairly similar to each other, they all have unique magnetic and electronic properties. These unique properties may stem from a phenomenon referred to as “lanthanide contraction.” The atomic and ionic radii of these elements are said to decrease despite the atomic number increasing, much like other groupings in the periodic table. As a result, the atoms become smaller as you move across the series. Scientists hadn’t experimentally observed this in all lanthanides in solution until now. The results of this groundbreaking research were recently published in Nature.
Unique Challenges of Handling Promethium
At any given time, there is usually only a little over a pound of this element in its naturally occurring state on Earth. Promethium is radioactive, but it has an incredibly short half-life. This plays a large role in its scarcity. The longest half-life of a promethium isotope, promethium-145, is only 17.7 years. ORNL was able to create a sample of promethium-147, which has a half-life of 2.6 years, using a by-product from the production of plutonium for space exploration. Once the clock starts ticking, the sample immediately starts to decay into the more stable element samarium.
A Rare Experiment with Promethium
“We had around 40 or 50% of the entire stock of purified promethium on the planet at the beamline to study,” remarked Bruce Ravel, lead beamline scientist at BMM and co-author of this research. “A couple of weeks later, the promethium sample was no longer usable, mostly due to the water in the solution evaporating. Of course, the research was interesting, but the whole process to make it happen was interesting, too. There was so much logistical planning and coordination necessary to make this happen, and everyone involved worked really hard to get every piece of this experiment in place quickly and carefully.”
The sample started at ORNL, where scientists extracted material from the waste stream of the High Flux Isotope Reactor and began to separate the promethium from the rest of the waste. Safely packaging the sample, driving it from Tennessee to New York, having it accepted at NSLS-II, and then carrying out experiments to the beamline all took time, and time takes away precious promethium.
Pioneering Research in Lanthanide Bonds
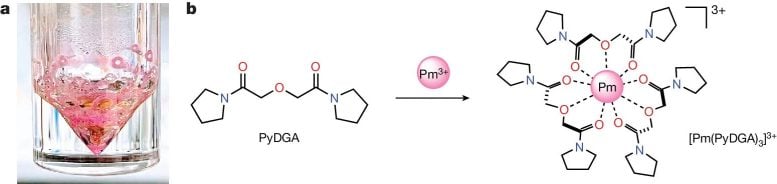
To study the chemical structure of promethium, the scientists had to first stabilize it in water. To do this, they used a water-soluble ligand called bispyrrolidine diglycolamide. Ligands are specialized molecules that bind to metal atoms. From there, the team took the sample to BMM to measure it using X-ray absorption spectroscopy (XAS), a well-established synchrotron technique that determines the structure and properties of atoms in a material by shining X-ray light on a sample and measuring how the components of that sample absorb the X-rays. Different atoms absorb X-rays at specific energies, which allows scientists to identify which elements are present and how they are arranged in the material.
“To the best of our knowledge, this was the first time that anyone, anywhere, at any synchrotron measured the element promethium with XAS,” remarked Ravel. “We are the first people ever to see a spectrum like that, which just by itself was really, really cool. I’ve been doing research using XAS for a long time, and I’ve never seen anything that no one else has ever seen before.”
In this solution, the promethium ion formed bonds with nine neighboring oxygen atoms. After analyzing and measuring the complex, the team was able to plug this result into the remaining lanthanide series, observing that it fit in the pattern of contraction that was theorized.
Acquiring this missing piece allowed the team to then analyze the series as a whole, which had its own interesting pattern. The bond shortening was fairly accelerated at the start of the series, but for the heavier lanthanides after promethium, the bond lengths shortened more steadily. Uncovering the chemical properties of promethium on its own opens a new realm of research possibilities, but gaining a more complete understanding of lanthanides completes a puzzle that has been missing a piece for quite some time.
“This was a situation where what we measured was in line with our expectations, which were informed by science and our knowledge of the lanthanide series,” said Ravel. “But we measured something extremely difficult that had never been measured before. Having actual knowledge, rather than inference, is how one does good science. The importance of filling in this gap in our collective knowledge of how lanthanides work is very important. I’ve been a scientist for 30 years and have never run into the street and yelled, ‘Eureka!’ This was an accomplishment, but it wasn’t a big surprise. The best part of science isn’t when someone says, ‘Eureka!’ It’s when someone says, ‘Huh, that’s weird.’”
Reference: “Observation of a promethium complex in solution” by Darren M. Driscoll, Frankie D. White, Subhamay Pramanik, Jeffrey D. Einkauf, Bruce Ravel, Dmytro Bykov, Santanu Roy, Richard T. Mayes, Lætitia H. Delmau, Samantha K. Cary, Thomas Dyke, April Miller, Matt Silveira, Shelley M. VanCleve, Sandra M. Davern, Santa Jansone-Popova, Ilja Popovs and Alexander S. Ivanov, 22 May 2024, Nature.
DOI: 10.1038/s41586-024-07267-6
Source link