Revolutionary Primate Study Reveals Critical Window for Alzheimer’s Treatment
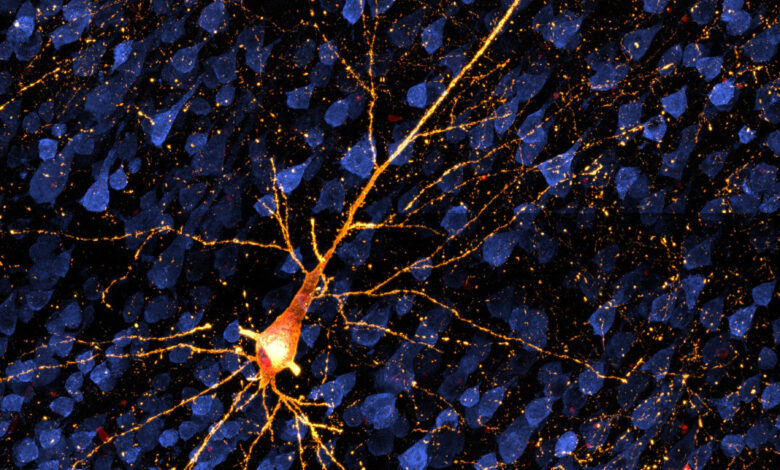

A dying neuron damaged by tau protein. Tau is involved in Alzheimer’s disease and other dementias. A new model in nonhuman primates is opening the possibility of testing treatments before extensive brain cell death and dementia set in. Credit: Danielle Beckman/UC Davis
Research in rhesus macaques has demonstrated a critical six-month window for monitoring and testing interventions for Alzheimer’s, focusing on the tau protein implicated in the disease. This study, revealing the progression of tau protein spread and its effects, enhances our understanding of early-stage Alzheimer’s and offers a bridge between rodent models and human clinical findings.
Research involving nonhuman primates is paving the way for trials of early-stage treatments for Alzheimer’s and related disorders, prior to significant brain cell loss and dementia. A study recently published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association indicates a six-month period during which the progression of the disease can be monitored and potential therapies can be evaluated in rhesus macaques.
“This is a very powerful translational model to test interventions that target the tau protein,” said John H. Morrison, professor of neurology at the University of California, Davis and California National Primate Research Center and corresponding author on the paper.
The tau protein is found in neurons in the brain. The spread of misfolded tau through the brain is implicated in Alzheimer’s disease, frontotemporal dementia, and other dementias. In Alzheimer’s, misfolded tau disrupts multiple processes essential for normal brain cell functioning. As the misfolded proteins spread, they affect neurons throughout the connected regions of the cortex that are crucial for memory and cognition.
The sick neurons then cause an inflammatory response mediated early on by microglial cells. Eventually, neurons die, leaving neurofibrillary tangles of tau protein, one of the key markers of Alzheimer’s and other dementing illnesses.
Thanks to advances in brain imaging, the discovery of biomarkers in human serum and cerebrospinal fluid, and work in rodent models, we now know more about the early stages of Alzheimer’s. But it is still difficult to figure out how tau, inflammation, and disease progression relate to each other.
The macaque model bridges the gap between what we can learn from mouse models and from human patients, said UC Davis postdoctoral researcher Danielle Beckman, first author on the paper.
Six months of measurable disease progression
The researchers injected a vector carrying DNA for two mutated tau proteins into the entorhinal cortex of 12 monkeys. The entorhinal cortex is a key brain region that is involved with memory and is where the disease usually originates in human Alzheimer’s.
Over six months, they followed the spread of tau protein, affected cells, and inflammation through the animals’ brains using PET and MRI imaging, biomarkers, and by microscopy.
“We can see tau pathology on neurons, and we can trace all the steps over a few months as the pathology spreads,” Beckman said.
The results show that in this model, there is a window of at least two to six months where the progress of the disease can be measured. That opens the possibility of preclinical testing of interventions that target the tau protein.
“We can look at drugs targeting early-stage Alzheimer’s before dementia develops,” Morrison said. “It’s all about early intervention to arrest progression.”
The paper builds on earlier work at the CNPRC establishing the nonhuman primate model. In future work, the researchers plan to combine the tau model with their existing model system based on amyloid.
Reference: “Temporal progression of tau pathology and neuroinflammation in a rhesus monkey model of Alzheimer’s disease” by Danielle Beckman, Giovanne B. Diniz, Sean Ott, Brad Hobson, Abhijit J. Chaudhari, Scott Muller, Yaping Chu, Akihiro Takano, Adam J. Schwarz, Chien-Lin Yeh, Paul McQuade, Paramita Chakrabarty, Nicholas M. Kanaan, Maria S. Quinton, Arthur A. Simen, Jeffrey H. Kordower and John H. Morrison, 21 June 2024, Alzheimer’s & Dementia.
DOI: 10.1002/alz.13868
The study was funded by the Alzheimer’s Association, the National Institutes of Health, NIH Office of the Director, and Takeda Pharmaceutical Company.
Additional authors on the study are: Giovanne Diniz, Sean Ott, Brad Hobson and Abhijit Chaudhari at UC Davis,; Scott Muller, Yaping Chu and Jeffrey Kordower, Arizona State University; Akihiro Takano, Adam Schwarz, Chien-Lin Yeh, Paul McQuade, Maria Quinton and Arthur Simen, Takeda Development Center Americas Inc., Lexington, Mass.; Paramita Chakrabarty, University of Florida; Nicholas Kanaan, Michigan State University.