Physicists Shed New Light on the “Invisible” Energy States of Molecules

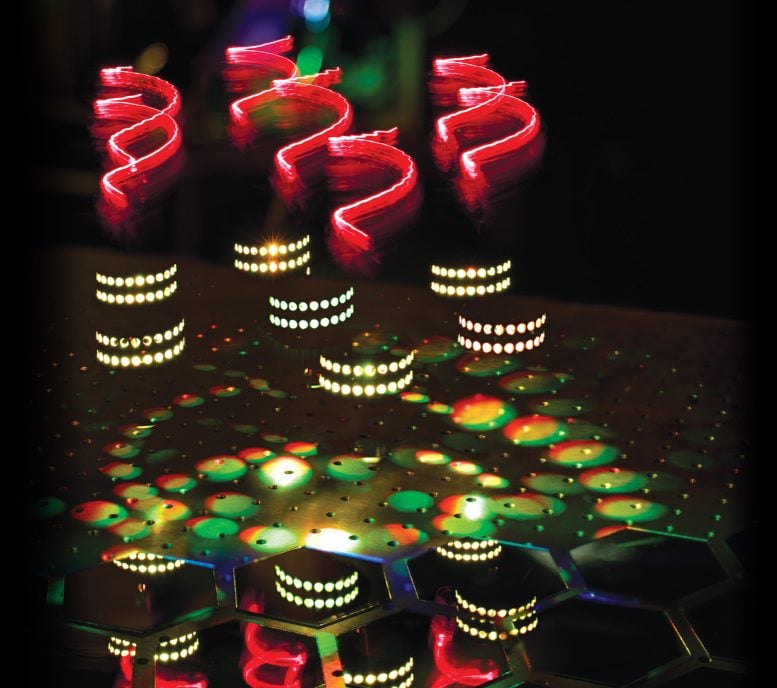
Artistic representation of hyper-Raman optical activity: twisted light (red helices) incident on molecules arranged on a helical scaffold (white dots) produce hyper-Raman scattering spectra (multicoloured light patches) that express ‘chirality’ (patches in spiral patterns and broken mirror). Credit: Ventsislav Valev and Kylian Valev
A team of scientists at the University of Bath in the UK has discovered a method to use light particles to uncover the hidden energy states of molecules.
An international team of scientists, led by physicists from the University of Bath, has demonstrated a new optical phenomenon that could significantly impact various fields, including pharmaceutical science, security, forensics, environmental science, art conservation, and medicine.
Molecules rotate and vibrate in very specific ways. When light shines on them it bounces and scatters. For every million light particles (photons), a single one changes colour. This change is the Raman effect. Collecting many of these color-changing photons paints a picture of the energy states of molecules and identifies them.
Yet some molecular features (energy states) are invisible to the Raman effect. To reveal them and paint a more complete picture, ‘hyper-Raman’ is needed.
Hyper-Raman
The hyper-Raman effect is a more advanced phenomenon than simple Raman. It occurs when two photons impact the molecule simultaneously and then combine to create a single scattered photon that exhibits a Raman color change.
Hyper-Raman can penetrate deeper into living tissue, it is less likely to damage molecules and it yields images with better contrast (less noise from autofluorescence). Importantly, while the hyper-Raman photons are even fewer than those in the case of Raman, their number can be greatly increased by the presence of tiny metal pieces (nanoparticles) close to the molecule.
Despite its significant advantages, so far hyper-Raman has not been able to study a key enabling property of life – chirality.
Optical activity
In molecules, chirality refers to their sense of twist – in many ways similar to the helical structure of DNA. Many bio-molecules exhibit chirality, including proteins, RNA, sugars, amino acids, some vitamins, some steroids and several alkaloids.
Light too can be chiral and in 1979, the researchers David L. Andrews and Thiruiappah Thirunamachandran theorized that chiral light used for the hyper-Raman effect could deliver three-dimensional information about the molecules, to reveal their chirality.
However, this new effect – known as hyper-Raman optical activity – was expected to be very subtle, perhaps even impossible to measure. Experimentalists who failed to observe it struggled with the purity of their chiral light. Moreover, as the effect is very subtle, they tried using large laser powers, but this ended up damaging the molecules being studied.
Explaining, Professor Ventsislav Valev who led both the Bath team and the study, said: “While previous attempts aimed to measure the effect directly from chiral molecules, we took an indirect approach. We employed molecules that are not chiral by themselves, but we made them chiral by assembling them on a chiral scaffold. Specifically, we deposited molecules on tiny gold nanohelices that effectively conferred their twist (chirality) to the molecules. The gold nanohelices have another very significant benefit – they serve as tiny antennas and focus light onto the molecules. This process augments the hyper-Raman signal and helped us to detect it. Such nanohelices were not featured on the 1979 theory paper and in order to account for them we turned to none other than one of the original authors and pioneer of this research field.”
Confirming a 45-year-old theory
Emeritus Professor Andrews from the University of East Anglia and co-author of the paper said: “It is very gratifying to see this work the experimental finally confirm our theoretical prediction, after all these years. The team from Bath have performed an outstanding experiment.”
This new effect could serve to analyze the composition of pharmaceuticals and to control their quality. It can help identify the authenticity of products and reveal fakes. It could also serve to identify illegal drugs and explosives at customs or crime scenes.
It will aid in detecting pollutants in environmental samples from air, water, and soil. It could reveal the composition of pigments in art for conservation and restoration purposes, and it will likely find clinical applications for medical diagnosis by detecting disease-induced molecular changes.
Professor Valev said: “This research work has been a collaboration between chemical theory and experimental physics across many decades and across academics of all stages – from PhD student to Emeritus Professor. We hope it will inspire other scientists and that it will raise awareness that scientific progress often takes many decades.”
Looking ahead he added: “Ours is the very first observation of a fundamental physical mechanism. There is a long way ahead until the effect can be implemented as a standard analytical tool that other scientist can adopt. We look forward to the journey, together with our collaborators from Renishaw PLC, a world-renowned manufacturer of Raman spectrometers.”
Dr Robin Jones, first-author for the new research paper and a PhD student at Bath until recently, said: “Performing the experiments that showed the hyper-Raman optical activity effect has been my most rewarding academic experience. In retrospect, it seems that almost every step of my PhD was like a piece of the puzzle which fell into place to achieve the observation.”
Reference: “Chirality conferral enables the observation of hyper-Raman optical activity” by Robin R. Jones, John F. Kerr, Hyunah Kwon, Samuel R. Clowes, Ruidong Ji, Emilija Petronijevic, Liwu Zhang, G. Dan Pantoș, Brian Smith, Tim Batten, Peer Fischer, Daniel Wolverson, David L. Andrews and Ventsislav K. Valev, 31 July 2024, Nature Photonics.
DOI: 10.1038/s41566-024-01486-z
The study was funded by The Royal Society, the Leverhulme Trust, and the Engineering and Physical Science Research Council (EPSRC).



