NIH Tests Experimental Monoclonal Antibody
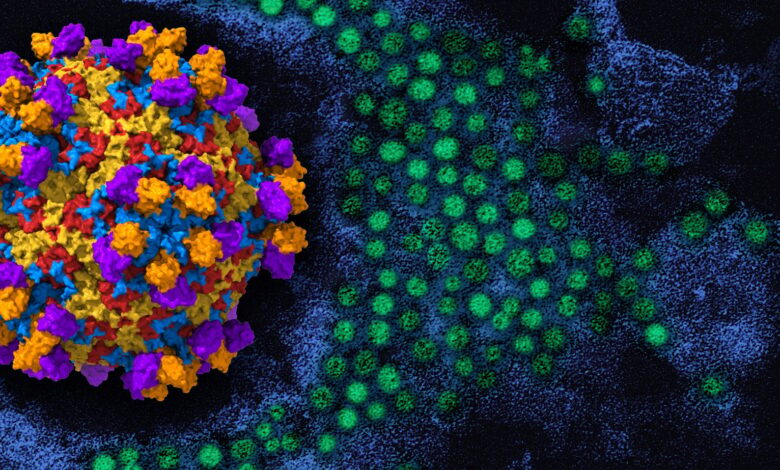

On the left is a 3D rendering of enterovirus D68 (viral proteins red, yellow, blue) with human monoclonal antibody EV68-228 (orange/purple). To the right in the background is a colorized transmission electron micrograph of enterovirus D68 virus particles (green). Credit: 3D rendering by NIAID; micrograph, repositioned and recolored by NIAID, courtesy of CDC.
A monoclonal antibody was developed from the blood of patients who were recovering.
The National Institutes of Health (NIH) is funding a clinical trial to assess the safety of a new monoclonal antibody aimed at treating enterovirus D68 (EV-D68). This virus can lead to severe respiratory and neurological conditions, including acute flaccid myelitis (AFM), which is similar to polio. Researchers are diligently working to deepen their understanding of AFM, which has seen periodic surges in the U.S., typically every two years during the late summer, over the past decade.
The U.S. Centers for Disease Control and Prevention (CDC) identified increases in AFM cases in 2014, 2016, and 2018. EV-D68 is a virus of growing public health concern due to its association with the intermittent AFM outbreaks.
There are no Food and Drug Administration-approved treatments for severe EV-D68 infection or AFM. Standard care is limited to supportive treatment and treatment for immune disorders, which has not been comprehensively evaluated. EV-D68 likely spreads from person to person when an infected person coughs, sneezes, or touches a surface that is then touched by others.
Development of a Potential Treatment
Between 2017 and 2019, scientists at Vanderbilt University Medical Center, Nashville, Tennessee, identified and isolated a neutralizing antibody, called EV68-228, from patients recovering from EV-D68 infection. Then, with collaborators from Utah State University, KBio, Inc., and ZabBio, the scientists developed an experimental antibody, called EV68-228-N, for testing. In laboratory models, the monoclonal antibody potently neutralized multiple clinical EV-D68 strains across multiple epidemic years. Kbio, Inc., is using its plant-based protein development platform to manufacture EV68-228-N.
Led by principal investigator C. Buddy Creech, M.D., M.P.H., at Vanderbilt University Medical Center, the Phase 1 study sponsored by NIH’s National Institute of Allergy and Infectious Diseases (NIAID) will evaluate the safety of EV68-228-N, how long it lasts in the body, and the most effective dose. The trial also will enroll participants at the University of Maryland, College Park, and be led by E. Adrianne Hammershaimb, M.D. The study is being conducted in collaboration with academic medical centers across the U.S. through the NIAID-funded Infectious Diseases Clinical Research Consortium, which includes the NIAID-funded Vaccine and Treatment Evaluation Units.
Monitoring and Historical Context of EV-D68
Clinical Trial NCT06444048 will enroll 36 healthy volunteers ages 18 to 49. Six will receive a placebo (control group) and 30—in groups of 10—will receive either a 3, 10, or 30 mg/kg dose of EV68-228-N intravenously. As part of the safety evaluation, scientists will monitor the first two study participants in each group receiving the experimental treatment for at least 72 hours before others receive the infusion. Researchers will then monitor and evaluate study participants during nine subsequent in-person examinations over the next 120 days.
According to the CDC, EV-D68 was first identified in California in 1962 and is one of more than 100 non-polio enteroviruses. EV-D68 typically causes respiratory illnesses that are mild. Non-polio enteroviruses are very common. Most infections are asymptomatic or cause mostly mild symptoms, such as runny nose, sneezing, cough, rash, mouth sores, body aches, and muscle aches. Severe symptoms may include wheezing and difficulty breathing.
Beginning in 1987, physicians and public health officials began reporting sporadic EV-D68 cases to CDC. However, between August and December 2014, EV-D68 caused an outbreak of respiratory illness in the U.S. and 120 cases of AFM in 34 states. This raised awareness of EV-D68-associated illness and, beginning in 2014, CDC surveillance for EV-D68 expanded. EV-D68 and cases of AFM have been subsequently detected in the U.S. every year, mostly in late summer and early fall, with pronounced spikes in 2016 and 2018.