Newly-Discovered Technique Turns Ordinary White Fat Cells Into Calorie-Burning Beige Fat


A UCSF study discovered that suppressing the protein KLF-15 can turn ordinary white fat cells into calorie-burning beige fat cells, a breakthrough that may explain why previous drug trials failed. This finding could pave the way for new, effective weight-loss drugs that convert white fat into beige fat, potentially offering a more sustainable method for combating obesity.
A new study reveals that inhibiting a protein can transform energy-storing white fat into calorie-burning beige fat, potentially paving the way for effective weight-loss drugs and explaining past clinical trial failures.
Researchers at UC San Francisco (UCSF) have discovered a method to change ordinary white fat cells, which typically store calories, into beige fat cells that burn calories to regulate body temperature.
The discovery could open the door to developing a new class of weight-loss drugs and may explain why clinical trials of related therapies have not been successful. Until now, researchers believed creating beige fat might require starting from stem cells. The new study, recently published in the Journal of Clinical Investigation, showed that ordinary white fat cells can be converted into beige fat simply by limiting the production of a protein.
“A lot of people thought this wasn’t feasible,” said Brian Feldman, MD, PhD, the Walter L. Miller, MD Distinguished Professor in Pediatric Endocrinology and senior author of the study. “We showed not only that this approach works to turn these white fat cells into beige ones, but also that the bar to doing so isn’t as high as we’d thought.”
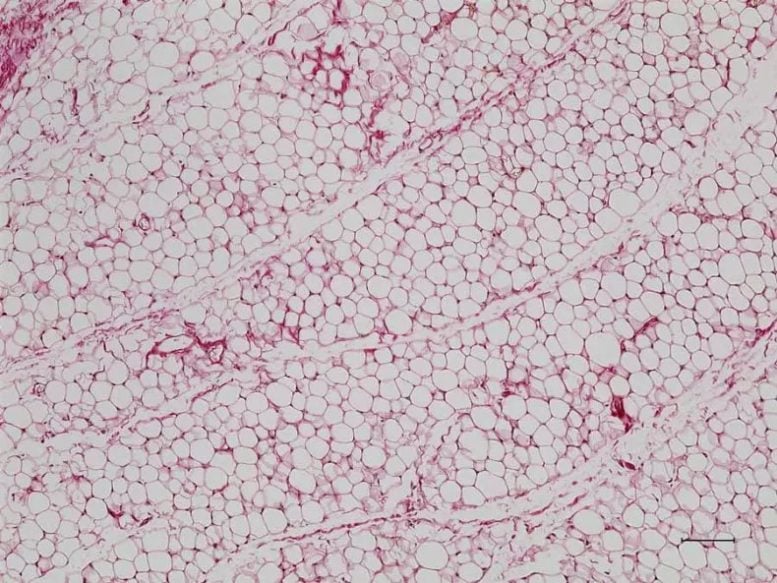
Ordinary white fat cells, which are common, store energy in a single, large fat droplet. Credit: Liang Li, Feldman Lab
A fat transformation
Many mammals have three “shades” of fat cells: white, brown, and beige. White fat serves as energy reserves for the body, while brown fat cells burn energy to release heat, which helps maintain body temperature. Beige fat cells combine these characteristics. They burn energy, and unlike brown fat cells, which grow in clusters, beige fat cells are embedded throughout white fat deposits. Humans and many other mammals are born with brown fat deposits that help them maintain body temperature after birth. But, while a human baby’s brown fat disappears in the first year of life, beige fat persists.
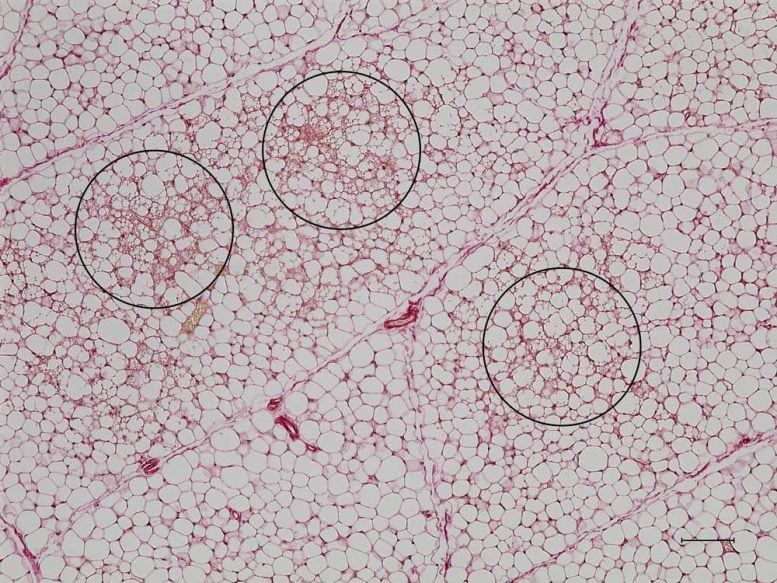
White fat cells without KLF-15 protein are converted to energy-burning beige fat cells (circled), which contain multiple small fat droplets. Credit: Liang Li, Feldman Lab
Humans can naturally turn white fat cells into beige ones in response to diet or a cold environment. Scientists tried to mimic this by coaxing stem cells into becoming mature beige fat cells. But stem cells are rare, and Feldman wanted to find a switch he could flip to turn white fat cells directly into beige ones.
“For most of us, white fat cells are not rare and we’re happy to part with some of them,” he said.
Of mice and humans
Feldman knew from his earlier experiments that a protein called KLF-15 plays a role in metabolism and the function of fat cells. With postdoctoral scholar Liang Li, PhD, Feldman decided to investigate how the protein functioned in mice, which retain brown fat throughout their lives. They found that KLF-15 was much less abundant in white fat cells than in brown or beige fat cells. When they then bred mice with white fat cells that lacked KLF-15, the mice converted them from white to beige. Not only could the fat cells switch from one form into another, but without the protein, the default setting appeared to be beige.
The researchers then looked at how KLF-15 exerts this influence. They cultured human fat cells and found that the protein controls the abundance of a receptor called Adrb1, which helps maintain energy balance. Scientists knew that stimulating a related receptor, Adrb3, caused mice to lose weight. But human trials of drugs that act on this receptor have had disappointing results.
A different drug targeting the Adrb1 receptor in humans is more likely to work, according to Feldman, and it could have significant advantages over the new, injectable weight-loss drugs that are aimed at suppressing appetite and blood sugar. Feldman’s approach might avoid side effects like nausea because its activity would be limited to fat deposits, rather than affecting the brain. And the effects would be long-lasting because fat cells are relatively long-lived.
“We’re certainly not at the finish line, but we’re close enough that you can clearly see how these discoveries could have a big impact on treating obesity,” he said.
Reference: “White adipocytes in subcutaneous fat depots require KLF15 for maintenance in preclinical models” by Liang Li and Brian J. Feldman, 1 July 2024, The Journal of Clinical Investigation.
DOI: 10.1172/JCI172360
This work was funded by NIH grant R01DK132404.



