Mysterious New Organism Found in Mono Lake Could Rewrite the History of Life

Berkeley scientists have discovered a new choanoflagellate species in Mono Lake that forms multicellular colonies and hosts a microbiome, offering new perspectives on the evolution of multicellular organisms.
The salty, arsenic- and cyanide-laced waters of the Eastern Sierra Nevada’s Mono Lake is an extremely hostile environment. Aside from the abundant brine shrimp and black clouds of alkali flies, very few organisms live there.
Now, researchers from the University of California, Berkeley have discovered a new creature lurking in the lake’s briny shallows — one that could tell scientists about the origin of animals more than 650 million years ago.
Discovering the Choanoflagellate
The organism is a choanoflagellate, a microscopic, single-celled form of life that can divide and develop into multicellular colonies in a way that’s similar to how animal embryos form. It’s not a type of animal, however, but a member of a sister group to all animals. As animals’ closest living relative, the choanoflagellate is a crucial model for the leap from one-celled to multicellular life.
Surprisingly, it harbors its own microbiome, making it the first choanoflagellate known to establish a stable physical relationship with bacteria, instead of solely eating them. As such, it’s one of the simplest organisms known to have a microbiome.
Choanoflagellates: Bridging the Gap in Evolution
“Very little is known about choanoflagellates, and there are interesting biological phenomena that we can only gain insight into if we understand their ecology,” said Nicole King, a UC Berkeley professor of molecular and cell biology and a Howard Hughes Medical Institute (HHMI) investigator who studies choanoflagellates as a model for what early life was like in ancient oceans.
Typically visible only through a microscope, choanoflagellates are often ignored by aquatic biologists, who instead focus on macroscopic animals, photosynthetic algae, or bacteria. But their biology and lifestyle can give insight into creatures that existed in the oceans before animals evolved and that eventually gave rise to animals. This species in particular could shed light on the origin of interactions between animals and bacteria that led to the human microbiome.
“Animals evolved in oceans that were filled with bacteria,” King said. “If you think about the tree of life, all organisms that are alive now are related to each other through evolutionary time. So if we study organisms that are alive today, then we can reconstruct what happened in the past.”
King and her UC Berkeley colleagues described the organism — which they named Barroeca monosierra, after the lake — in a paper published in the journal mBio.
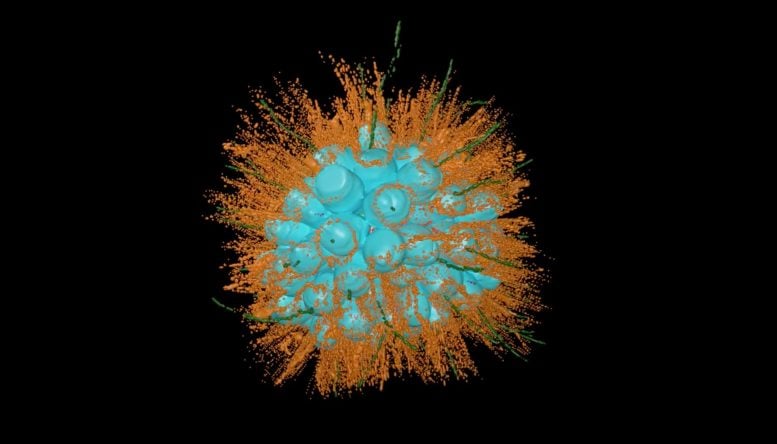
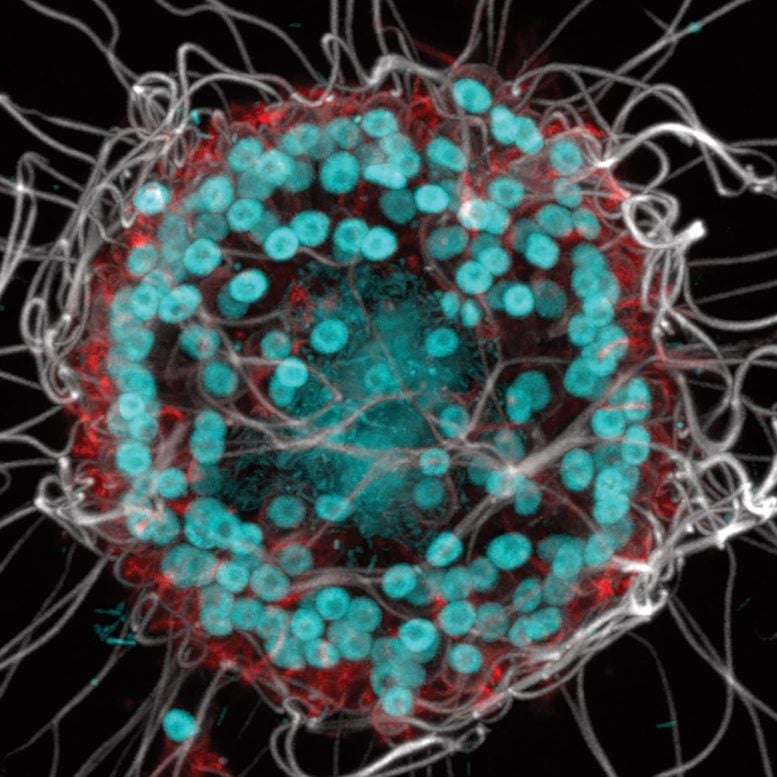
The newly named species Barroeca monosierra discovered in Mono Lake. Colonies of these organisms consist of numerous identical cells (cyan), each with flagella (green) that allow them to propel themselves through the water. This choanoflagellate colony hosts its own microbiome (red), something never before seen in these organisms. The extracellular matrix with which the bacteria interact is shown in white. Credit: Davis Laundon and Pawel Burkhardt, Sars Centre, Norway; Kent McDonald and Nicole King, UC Berkeley
Insights from a Surprising Find
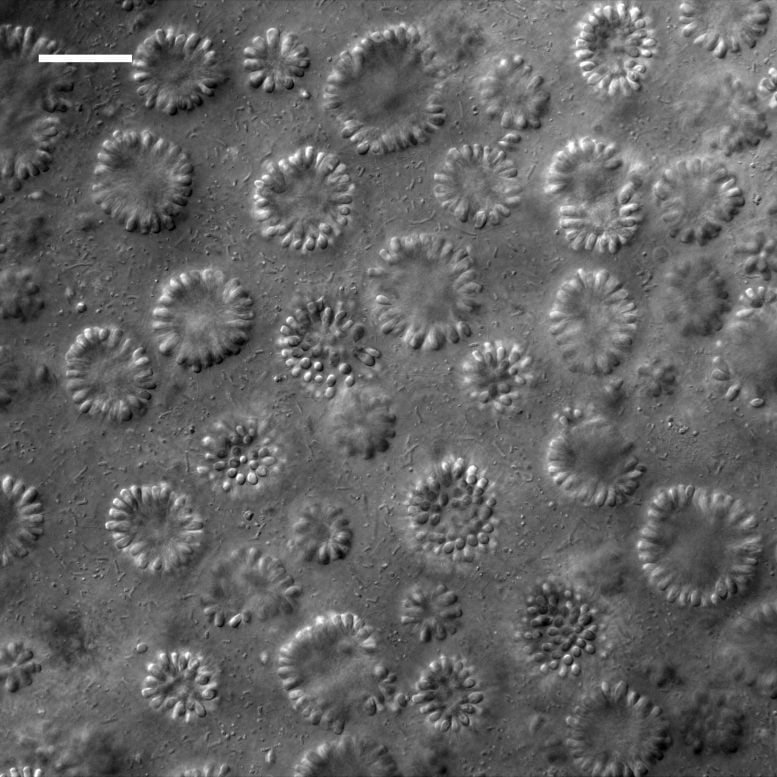
Nearly 10 years ago, then-UC Berkeley graduate student Daniel Richter came back from a climbing trip in the Eastern Sierra Nevada with a vial of Mono Lake water he’d casually collected along the way. Under the microscope, it was alive with choanoflagellates. Other than brine shrimp, alkali flies and various species of nematode, few other forms of life have been reported to live in the inhospitable waters of the lake.
“It was just packed full of these big, beautiful colonies of choanoflagellates,” King said. “I mean, they were the biggest ones we’d ever seen.”
The colonies of what seemed to be close to 100 identical choanoflagellate cells formed a hollow sphere that twirled and spun as each individual cell kicked its flagella.
“One of the things that’s interesting about them is that these colonies have a shape similar to the blastula — a hollow ball of cells that forms early in animal development,” King said. “We wanted to learn more about it.”
At the time, however, King was occupied with other species of choanos, as she calls them, so the Mono Lake choanos languished in the freezer until some students revived and stained them to look at their unusual, doughnut-shaped chromosomes. Surprisingly, there was also DNA inside the hollow colony where there should have been no cells. After further investigation, graduate student Kayley Hake determined that they were bacteria.
The Study of Choanoflagellates and Bacteria
“The bacteria were a huge surprise. That just was fascinating,” King said.
Hake also detected connective structures, called extracellular matrix, inside the spherical colony that were secreted by the choanos. Only then did it occur to Hake and King that these might not be the remains of bacteria the choanos ate, but bacteria living and grazing on stuff secreted by the colony.
“No one had ever described a choanoflagellate with a stable physical interaction with bacteria,” she said. “In our prior studies, we found that choanos responded to small bacterial molecules that were floating through the water, or [that] the choanos were eating the bacteria, but there was no case where they were doing anything that could potentially be a symbiosis. Or in this case, a microbiome.”
Future Research and Implications
King teamed up with Jill Banfield, a pioneer in metagenomics and a UC Berkeley professor of environmental science, policy and management and of earth and planetary science, to determine which bacterial species were in the water and inside the choanos. Metagenomics involves sequencing all the DNA in an environmental sample to reconstruct the genomes of the organisms living there.
After Banfield’s lab identified the microbes in Mono Lake water, Hake created DNA probes to determine which ones were also inside the choanos. The bacterial populations were not identical, King said, so evidently some bacteria survive better than others inside the oxygen-starved lumen of the choanoflagellate colony. Hake determined that they were not there accidentally; they were growing and dividing. Perhaps they were escaping the toxic environment of the lake, King mused, or maybe the choanos were farming the bacteria to eat them.
Much of this is speculation, she admits. Future experiments should uncover how the bacteria interact with the choanoflagellates. Past work in her lab has already shown that bacteria act like an aphrodisiac to stimulate mating in choanoflagellates, and that bacteria can stimulate single-celled choanos to aggregate into colonies.
For her, the Mono Lake choanoflagellate will become another model system in which to study evolution, just like the choanos that live in splash pools on the island of Curaçao in the Caribbean — her main focus at the moment — and the choanos in pools at the North and South Poles. It might be a challenge to get more samples from Mono Lake, however. On a recent visit, only six of 100 samples contained these energetic microorganisms.
“I think there’s a great deal more that needs to be done on the microbial life of Mono Lake, because it really underpins everything else about the ecosystem,” King said. “I’m excited about B. monosierra as a new model for studying interactions between eukaryotes and bacteria. And I hope it tells us something about evolution. But even if it doesn’t, I think it’s a fascinating phenomenon.”
Reference: “A large colonial choanoflagellate from Mono Lake harbors live bacteria” by K. H. Hake, P. T. West, K. McDonald, D. Laundon, J. Reyes-Rivera, A. Garcia De Las Bayonas, C. Feng, P. Burkhardt, D. J. Richter, J. F. Banfield and N. King, 14 August 2024, mBio.
DOI: 10.1128/mbio.01623-24
The work is supported by HHMI and the National Science Foundation.
Source link



