Mapping the Molecular Mayhem Caused by Huntington’s Disease
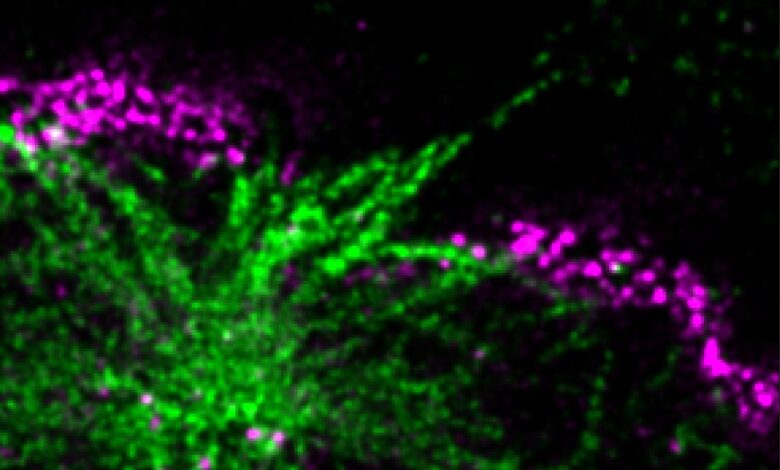
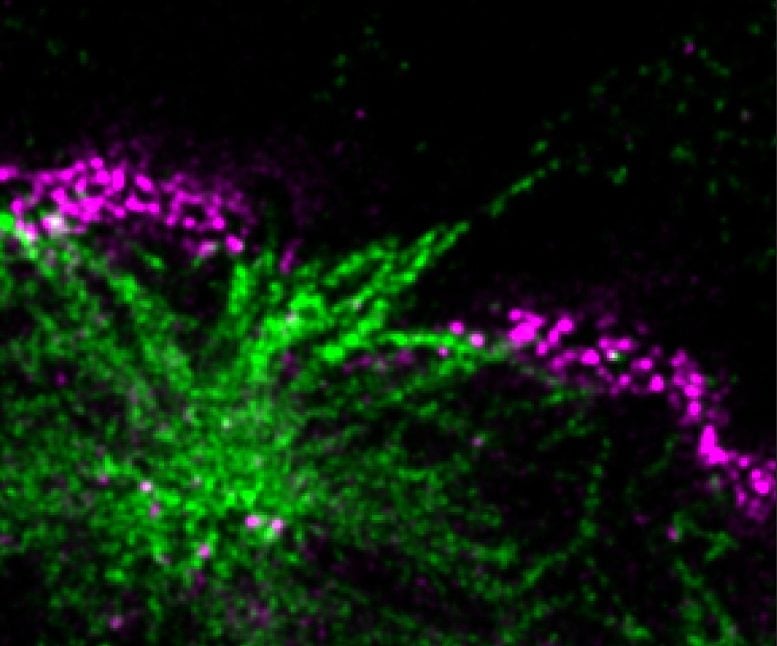
Researchers have uncovered a new mechanism by which Huntington’s disease causes neuronal death.
They found that toxic protein aggregates from a mutated huntingtin protein can damage the nuclear envelope, leading to DNA damage and gene misregulation in neurons. This breakthrough could link similar mechanisms to other neurodegenerative diseases, suggesting a common pathway for neuronal damage and offering potential new targets for therapeutic intervention.
Toxic Protein Aggregates in Huntington’s Disease
Scientists at Utrecht University in the Netherlands have identified a new way in which the toxic protein aggregates associated with Huntington’s disease may damage nerve cells and cause them to die. The study, to be published today (August 16) in the Journal of Cell Biology (JCB), suggests that the aggregates can poke holes in the membrane that separates the nucleus from the rest of the cell, damaging the DNA inside the nucleus and changing the activity of neuronal genes.
Huntington’s disease is a devastating neurogenerative disorder caused by a mutation in the HTT gene that results in cells producing abnormally large versions of the huntingtin protein. These expanded huntingtin proteins aggregate inside cells and damage them in various ways, although exactly how this results in the death of nerve cells remains uncertain.
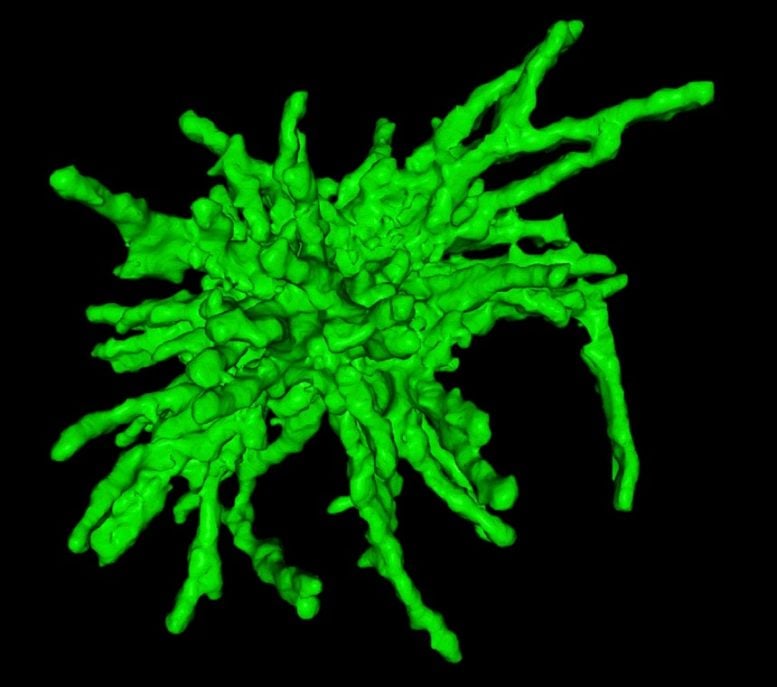
New Insights Into Neuronal Damage
Spearheaded by graduate student Giel Korsten from the group of Lukas Kapitein, the researchers discovered a major new way in which huntingtin aggregates damage cells when they examined neurons expressing the expanded version of the protein. The researchers found that many of the nerve cells had breaks in the membrane that separates the nucleus from the rest of the cell. This barrier, known as the nuclear envelope, protects and regulates the chromosomes inside the nucleus, allowing them to turn genes on and off as needed.
Kapitein and colleagues found that huntingtin aggregates inside the nucleus disrupt the protein meshwork that underlies and strengthens the nuclear envelope, making the membrane more likely to rupture. Using a specialized technique known as expansion microscopy to visualize the nuclear aggregates in high detail, the researchers saw that tiny fibrils stick out from the aggregates and poke through the meshwork underlying the nuclear envelope. The aggregates may also impair the cell’s ability to reseal the envelope once it breaks, the researchers found.
Implications of Nuclear Envelope Ruptures
“We have discovered that the aggregates associated with Huntington’s disease induce ruptures in the nuclear envelope that compromise its barrier function,” Kapitein says.
Over time, these disruptions in the nuclear envelope likely lead to damage of the nerve cell’s DNA and the misregulation of neuronal genes, cellular defects that have previously been linked to Huntington’s disease pathology.
Broader Impact on Neurodegenerative Diseases
Kapitein notes that several other neurogenerative diseases, including certain types of amyotrophic lateral sclerosis and frontotemporal dementia, are associated with the formation of protein aggregates inside the cell nucleus. “We speculate that nuclear aggregate–induced ruptures in the nuclear envelope represent a common contributor to neurodegeneration that initiates a cascade of deregulated processes culminating in neuronal death and neuroinflammation,” Kapitein says.
Reference: “Nuclear poly-glutamine aggregates rupture the nuclear envelope and hinder its repair” 16 August 2024, Journal of Cell Biology.
DOI: 10.1083/jcb.202307142
Source link