Existing Cancer Drug Alleviates Rare Genetic Syndrome

A recent study shows that ruxolitinib, a drug targeting interferon-gamma (IFN-gamma), effectively alleviates symptoms of the rare autoimmune polyendocrine syndrome type 1 (APS-1) in both mice and humans, paving the way for new treatments for this and related diseases. 3D model of the drug ruxolitinib. Credit: NIAID
Researchers at the NIH have discovered new pathways that could lead to treatments for autoimmune polyendocrine syndrome type 1.
A medication originally designed for some autoimmune disorders and cancers has been effective in treating symptoms of a rare genetic condition known as autoimmune polyendocrine syndrome type 1 (APS-1). This finding emerged after researchers discovered that the syndrome correlates with increased levels of interferon-gamma (IFN-gamma), a protein that plays a role in immune responses.
This discovery sheds light on the role of IFN-gamma in autoimmune diseases. The research, conducted by the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, was published in the New England Journal of Medicine.
In a three-stage study, conducted in mice and people, the researchers examined how APS-1 causes autoimmune disease. The syndrome is marked by dysfunction of multiple organs, usually beginning in childhood, and is fatal in more than 30% of cases. This inherited syndrome is caused by a deficiency in a gene that keeps the immune system’s T cells from attacking cells of the body, leading to autoimmunity; chronic yeast infections in the skin, nails, and mucous membranes; and insufficient production of hormones from endocrine organs, such as the adrenal glands. Symptoms include stomach irritation, liver inflammation, lung irritation, hair loss, loss of skin coloring, tissue damage, and organ failure.
Research Findings and Drug Testing
In the first stage of this study, researchers led by scientists in NIAID’s Laboratory of Clinical Immunology and Microbiology examined the natural history of APS-1 in 110 adults and children. Blood and tissues were analyzed to compare gene and protein expression in people with and without APS-1. They found elevated IFN-gamma responses in the blood and tissues of people with APS-1, indicating that IFN-gamma may play an important role in the disease and providing a pathway to target for treatment.
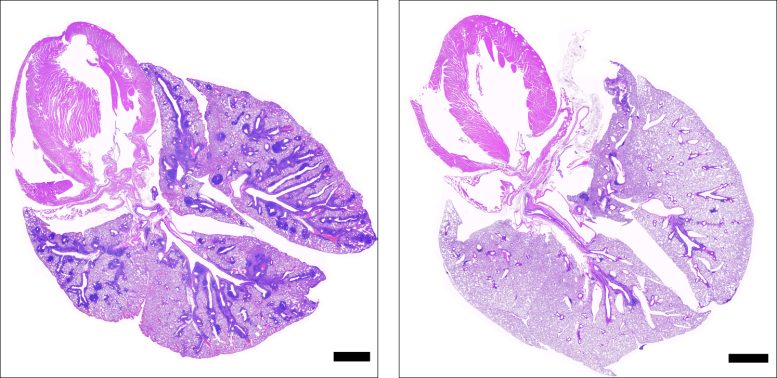
Cross-sections of lungs from mice with the gene deficiency that causes APS-1, showing damaged tissue in mice not administered ruxolitinib (left) and healthy tissue in mice administered ruxolitinib (right). Black bars represent 1 mm. Credit: NIAID
In the second stage of the study, the scientists examined mice with the same gene deficiency that causes APS-1 in people, finding that the animals also experienced autoimmune tissue damage and elevated IFN-gamma levels. Mice also deficient in the gene for IFN-gamma did not have autoimmune tissue damage, which showed a direct link between IFN-gamma and APS-1 symptoms.
With this understanding, the researchers looked for a drug that could be used to lower IFN-gamma activity in people. They selected ruxolitinib, a Janus kinase inhibitor, because it acts by shutting down the pathway driven by IFN-gamma. When ruxolitinib was administered to the mice with the gene deficiency that causes APS-1, IFN-gamma responses were normalized and T cells were prevented from infiltrating tissues and damaging organs. These results showed that ruxolitinib could alleviate the effects of the gene deficiency, suggesting that it could be effective for the treatment of APS-1 in people.
The researchers administered ruxolitinib, which was supplied by the NIH Clinical Center, to five people—two adults and three children—with APS-1 in the third stage of the study. The dosing and regimens were tailored to the individuals, and the treatments were continued for over a year. The drug was safe and tolerated well, and improvement in symptoms was seen in all study participants. Blood and tissue analyses revealed decreased production of IFN-gamma from T cells, as well as normalized levels of IFN-gamma in the blood. Many APS-1-related symptoms were reduced, including hair loss, oral yeast infections, stomach and bowel irritation, hives, and thyroid inflammation.
The results revealed that normalizing IFN-gamma levels using ruxolitinib could reduce the damaging effects of APS-1 in people. The scientists note that a study with a larger and more diverse group of patients is needed to determine whether ruxolitinib and similar drugs are suitable treatments for individuals with APS-1. They write that understanding the role of IFN-gamma in autoimmunity may lead to the development of treatments for related diseases. This research highlights the importance of finding the causes of and treatments for rare diseases.
Reference: “The Role of Interferon-γ in Autoimmune Polyendocrine Syndrome Type 1” by Vasileios Oikonomou, Grace Smith, Gregory M. Constantine, Monica M. Schmitt, Elise M.N. Ferré, Julie C. Alejo, Deanna Riley, Dhaneshwar Kumar, Lucas Dos Santos Dias, Joseph Pechacek, Yannis Hadjiyannis, Taura Webb, Bryce A. Seifert, Rajarshi Ghosh, Magdalena Walkiewicz, Daniel Martin, Marine Besnard, Brendan D. Snarr, Shiva Deljookorani, Chyi-chia R. Lee, Tom DiMaggio, Princess Barber, Lindsey B. Rosen, Aristine Cheng, Andre Rastegar, Adriana A. de Jesus, Jennifer Stoddard, Hye Sun Kuehn, Timothy J. Break, Heidi H. Kong, Leslie Castelo-Soccio, Ben Colton, Blake M. Warner, David E. Kleiner, Martha M. Quezado, Jeremy L. Davis, Kevin P. Fennelly, Kenneth N. Olivier, Sergio D. Rosenzweig, Anthony F. Suffredini, Mark S. Anderson, Marc Swidergall, Carole Guillonneau, Luigi D. Notarangelo, Raphaela Goldbach-Mansky, Olaf Neth, Maria Teresa Monserrat-Garcia, Justo Valverde-Fernandez, Jose Manuel Lucena, Ana Lucia Gomez-Gila, Angela Garcia Rojas, Mikko R. J. Seppänen, Jouko Lohi, Matti Hero, Saila Laakso, Paula Klemetti, Vanja Lundberg, Olov Ekwall, Peter Olbrich, Karen K. Winer, Behdad Afzali, Niki M. Moutsopoulos, Steven M. Holland, Theo Heller, Stefania Pittaluga and Michail S. Lionakis, 29 May 2024, New England Journal of Medicine.
DOI: 10.1056/NEJMoa2312665
The research was funded by the National Institute of Allergy and Infectious Diseases.



