Cellular Railroads Guide Healing in the Human Body

Cells in the human body typically cannot move, but certain types can travel to repair tissues, notably during wound healing. Researchers investigating cell movement in vitro, discovered that cells often travel in formations resembling trains, initially sticking together and then forming groups that move along predefined tracks. Credit: SciTechDaily.com
Researchers discovered that specific cells in the human body travel in trains or clusters to repair tissues, guided by a feature called polarity.
Looking under the microscope, a group of cells slowly moves forward in a line, like a train on the tracks. The cells navigate through complex environments. A new approach by researchers involving the Institute of Science and Technology Austria (ISTA) now shows how they do this and how they interact with each other.
Cells on the Move
The majority of the cells in the human body cannot move. Some specific ones, however, can go to different places. For example, in wound healing, cells move through the body to repair damaged tissue. They sometimes travel alone or in different group sizes. Although the process is increasingly understood, little is known about how cells interact while traveling and how they collectively navigate the complex environments found in the body. An interdisciplinary team of theoretical physicists at the Institute of Science and Technology Austria (ISTA) and experimentalists from the University of Mons in Belgium now has new insights.
Much like social dynamics experiments, where understanding the interactions of a small group of people is easier than analyzing an entire society, the scientists studied the traveling behavior of a small group of cells in well-defined in vitro surroundings, i.e. outside a living organism, in a Petri dish equipped with interior features. Based on their findings, they developed a framework of interaction rules, which is published today (June 19) in Nature Physics.
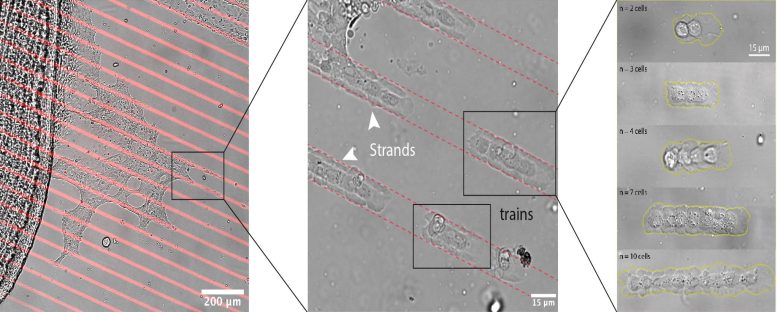
Snapshots of the cell railroad. Cells stretch away from a fish scale (left) into artificial lanes (red) and form trains (middle) in different sizes (right). Credit: © Vercurysse, Brückner et al./Nature Physics
Cells Travel in Trains
David Brückner rushes back to his office to grab his laptop. “I think it’s better to show some videos of our experiments,” he says excitedly and presses play.
The video shows a Petri dish. Microstripes—one-dimensional lanes guiding cell movement—are printed on the substrate beside a zebrafish scale made up of numerous cells. Special wound-healing cells, known as “keratocytes” start to stretch away from the scale, forming branches into the lanes. “At first, cells stick together through adhesive molecules on their surface—it’s like they’re holding hands,” explains Brückner. Suddenly, the bond breaks off, and the cells assemble into tiny groups, moving forward like trains along tracks. “The length of the train is always different. Sometimes it’s two, sometimes it’s ten. It depends on the initial conditions.”
Eléonore Vercurysse and Sylvain Gabriele from the University of Mons in Belgium observed this phenomenon while investigating keratocytes and their wound-healing features within different geometrical patterns. To help interpret these puzzling observations, they reached out to theoretical physicists David Brückner and Edouard Hannezo at ISTA.

Inspiration on the blackboard. Edouard Hannezo (back) and David Brückner (front) brainstorm mathematical equations. They use one of the many blackboards found all over the ISTA campus, which allows for spontaneous ideas to flow and be exchanged. Credit: © ISTA
Cells Have a Steering Wheel
“There’s a gradient within each cell that determines where the cell is going. It’s called ‘polarity’ and it’s like the cell’s very own steering wheel,” says Brückner. “Cells communicate their polarity to neighboring cells, allowing them to move in concert.” But how they do so has remained a big puzzle in the field.
Brückner and Hannezo started brainstorming. The two scientists developed a mathematical model combining a cell’s polarity, their interactions, and the geometry of their surroundings. They then transferred the framework into computer simulations, which helped them visualize different scenarios.
The first thing the scientists in Austria looked at was the speed of the cell trains. The simulation revealed that the speed of the trains is independent of their length, whether they consist of two or ten cells. “Imagine if the first cell did all the work, dragging the others behind it; the overall performance would decrease,” says Hannezo. “But that’s not the case. Within the trains, all the cells are polarized in the same direction. They are aligned and in sync about their movement and smoothly move forward.” In other words, the trains operate like an all-wheel drive rather than just a front-wheel drive.
As a next step, the theoreticians examined the effects of increasing the width of the lanes and the cell clusters in their simulations. Compared to cells moving in a single file, clusters were much slower. The explanation is quite simple: the more cells are clustered together, the more they bump into each other. These collisions cause them to polarize away from each other and move in opposite directions. The cells are not aligned properly, which disrupts the flow of movement and drastically influences the overall speed. This phenomenon was also observed in the Belgian lab (in vitro experiments).
Dead End? No Problem for Cell Clusters
From an efficiency standpoint, it sounds like moving in clusters is not ideal. However, the model predicted that it also had its benefits when cells navigate through complex terrain, as they do, for instance, in the human body. To test this, the scientists added a dead end, both in the experiments and in the simulations. “Trains of cells get to the dead end quickly, but struggle to change direction. Their polarization is well aligned, and it’s very hard for them to agree on switching around,” says Brückner. “Whereas in the cluster, quite a few cells are already polarized in the other direction, making the change of direction way easier.”
Trains or Clusters?
Naturally, the question arises: when do cells move in clusters, and when do they move in trains? The answer is that both scenarios are observed in nature. For example, some developmental processes rely on clusters of cells moving from one side to the other, while others depend on small trains of cells moving independently. “Our model doesn’t only apply to a single process. Instead, it is a broadly applicable framework showing that placing cells in an environment with geometric constraints is highly instructive, as it challenges them and allows us to decipher their interactions with each other,” Hannezo adds.
A Small Train Packed With Information
Recent publications by the Hannezo group suggest that cell communication propagates in waves—an interplay between biochemical signals, physical behavior, and motion. The scientists’ new model now provides a physical foundation for these cell-to-cell interactions, possibly aiding in understanding the big picture. Based on this framework, the collaborators can delve deeper into the molecular players involved in this process. According to Brückner, the behaviors revealed by these small cell trains can help us understand large-scale movements, such as those seen in entire tissues.
Reference: “Geometry-driven migration efficiency of autonomous epithelial cell clusters” 19 June 2024, Nature Physics.
DOI: 10.1038/s41567-024-02532-x
Information on Animal Studies
In order to better understand fundamental processes, for example, in the fields of neuroscience, immunology, or genetics, the use of animals in research is indispensable. No other methods, such as in silico models, can serve as alternative. The animals are raised, kept, and treated according to the strict regulations of the respective countries, the research was conducted (Belgium).



