Pioneering Research Redefines Our Understanding of Cellular Aging

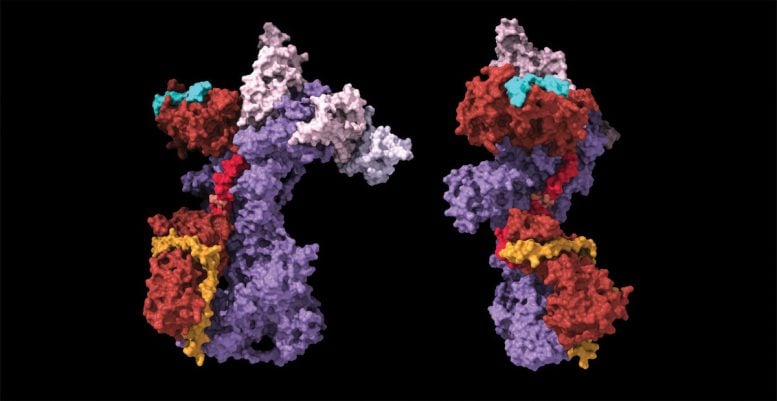
Recent discoveries in telomere biology reveal that the length and health of chromosome ends are regulated by enzymes telomerase and CST–Polα/primase, coordinated by the protein POT1, highlighting implications for treating telomere disorders and cancer.
The length of telomeres, which protect the ends of our chromosomes, must be carefully regulated. If they are too long, there is an increased risk of cancer; if they are too short, they lose their protective function, leading to telomere disorders that can have severe health implications.
Our cells prevent this excessive shortening by adding telomeric DNA to the ends of chromosomes. Researchers at Rockefeller recently showed that this process is mediated by two enzymes: telomerase and the CST–Polα/primase complex. Having determined how telomerase is recruited, scientists were left with a fundamental question: how does CST–Polα/primase find its way to the telomere?
Now, a new study published in Cell demonstrates that CST is recruited to the end of the telomere and regulated by subtle chemical changes made to POT1, a protein in the shelterin complex involved in telomere maintenance and implicated in cancer risk. The findings provide new insight into how human telomeres function at the molecular level, with implications for numerous diseases and disorders.
“After the discovery of telomerase, it took decades to figure out how it gets to the telomere. Now, just months after discovering that CST–Polα/primase is the second critical enzyme required for telomere maintenance, we understand the details of how it is recruited,” says Titia de Lange, the Leon Hess professor. “Moreover, we’ve found out how this process is regulated.”
Recruiting and regulating CST
Telomeres have two different types of strands, G-rich and C-rich. Scientists have long known how telomerase maintains the length of the G-rich strand, but only recently was it recognized that the same problem also exists for the C-rich strand. A recent study from the de Lange lab identified the CST–Polα/primase complex as the key regulator responsible for keeping that strand intact.
What remained to be seen was how CST, and its associated enzyme Polα-primase, travels to telomere to facilitate C-strand maintenance across replication cycles. Sarah Cai, a PhD candidate at Rockefeller, began investigating this piece of the telomere puzzle. Building on a decade of the de Lange lab’s groundwork on CST, Cai added cryo-EM to the techniques used in this study while being co-advised by Rockefeller’s Thomas Walz.
“The interdisciplinary nature of the study is one of the most exciting parts,” Cai says. “It was a very successful double-lab effort, making use of many different technologies.” Walz, whose research focuses on cryo-EM, noted how Cai incorporated AlphaFold, a deep-learning algorithm that can predict the unique 3D structures of proteins, into her work.
With the combined power of biochemistry, structural biology, and cell biology, the team ultimately confirmed that CST is recruited to telomeres by the POT1 protein. Once CST–Polα/primase is onsite, the addition and removal of phosphate groups from POT1 appears to function as an on/off switch that coordinates the final steps of telomere replication. Phosphorylated POT1 ensures that CST–Polα/primase remains inactive until telomerase has finished its job, upon which the dephosphorylation of POT1 activates CST–Polα/primase to add the finishing touches to the telomere.
Telomere disorders and cancer
Next, the team will look for specific enzymes that attach and remove phosphates during this process, controlling the on/off switch on POT1, and determining their role in regulating CST–Polα/primase recruitment and activity. A better understanding of how CST is recruited to the telomere cannot come fast enough for patients suffering from telomere disorders, such as Coats plus syndrome, a severe multi-organ disease characterized by abnormalities in the eyes, brain, bones, and GI tract.
“For a long time, we didn’t know why mild alterations in single amino acids would cause such a devastating disease,” Cai says. “We now have a better idea of how these mutations affect the recruitment of this critical telomere maintenance machine and lead to Coats plus syndrome.”
The findings will also impact their cancer research. The de Lange lab has spent decades studying how telomere shortening contributes to tumor suppression and genome instability in cancer, and the present research may ultimately help answer questions that lie at the heart of tumor development.
“Anything critical to telomere length regulation may well be critical to cancer prevention too,” de Lange says. “This is a major focus of our lab, and one of the reasons we’ll be looking into the interplay between CST–Polα/primase and telomerase more closely in the future.”
Reference: “POT1 recruits and regulates CST-Polα/primase at human telomeres” by Sarah W. Cai, Hiroyuki Takai, Arthur J. Zaug, Teague C. Dilgen, Thomas R. Cech, Thomas Walz and Titia de Lange, 4 June 2024, Cell.
DOI: 10.1016/j.cell.2024.05.002
Source link



