Meet the Microscopic Thieves Fighting Infections With Stolen Genes

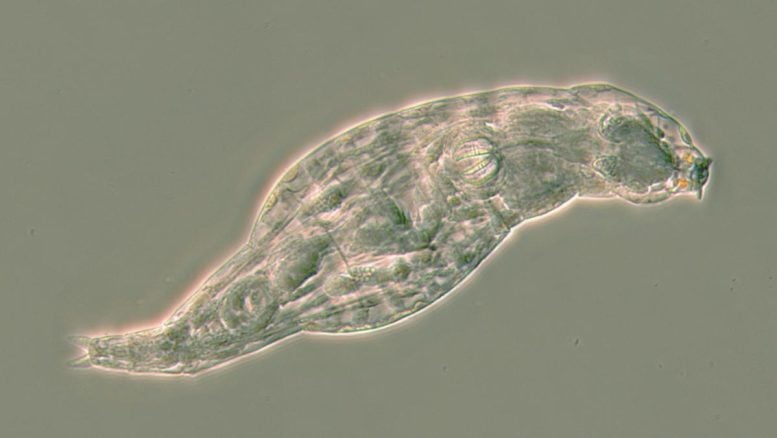
Bdelloid rotifers, a type of small freshwater animal, harness stolen bacterial genes to create antibiotics, offering insights into developing safer antimicrobial drugs and addressing growing antibiotic resistance.
A team of researchers from the University of Oxford, the University of Stirling, and the Marine Biological Laboratory (MBL), Woods Hole discovered that a group of tiny, freshwater animals protect themselves from infections using antibiotic recipes “stolen” from bacteria.
These microscopic creatures are called bdelloid rotifers, which means ‘crawling wheel-animals’. Although they are smaller than a hair’s breadth, they have a head, mouth, gut, muscles, and nerves like other animals.
Genetic Defense Mechanisms
The study, recently published in Nature Communications, reveals that when these rotifers are exposed to fungal infection, they activate hundreds of genes that they acquired from bacteria and other microbes. Some of these genes produce resistance weapons, such as antibiotics and other antimicrobial agents, in the rotifers.
“When we translated the DNA code to see what the stolen genes were doing, we had a surprise,” said lead study author Chris Wilson of the University of Oxford. “The main genes were instructions for chemicals that we didn’t think animals could make — they looked like recipes for antibiotics.”
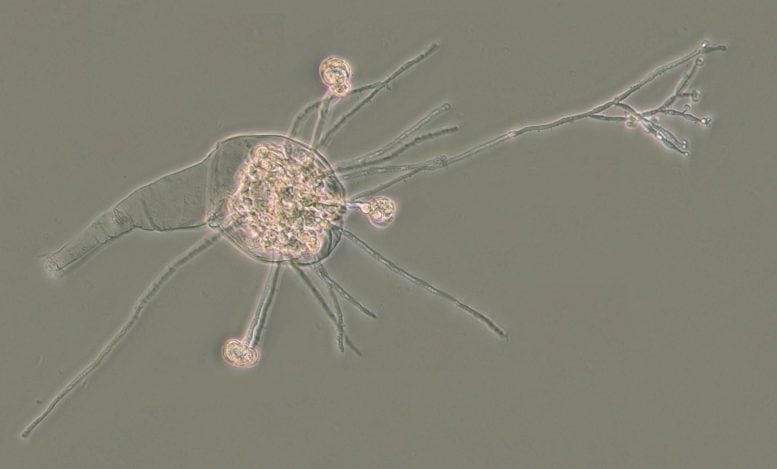
Prior research found that rotifers have been picking up DNA from their surroundings for millions of years, but the new study is the first to discover them using these genes against diseases. No other animals are known to “steal” genes from microbes on such a large scale.
“These complex genes – some of which aren’t found in any other animals – were acquired from bacteria but have undergone evolution in rotifers,” said study co-author David Mark Welch, senior scientist and director of the Josephine Bay Paul Center at the Marine Biological Laboratory. “This raises the potential that rotifers are producing novel antimicrobials that may be less toxic to animals, including humans, than those we develop from bacteria and fungi.”
Unveiling Unique Antibiotic Production
Antibiotics are essential to modern healthcare, but most of them were not invented by scientists. Instead, they are produced naturally by fungi and bacteria in the wild, and humans can make artificial versions to use as medicine.
The new study suggests that rotifers might be doing something similar.
“These strange little animals have copied the DNA that tells microbes how to make antibiotics,” explains Wilson. “We watched them using one of these genes against a disease caused by a fungus, and the animals that survived the infection were producing 10 times more of the chemical recipe than the ones that died, indicating that it helps to suppress the disease.”
This bdelloid rotifer is using her wheels to hoover up and eat bacteria and other particles floating in the water. This animal is about half a millimeter long, and the wheels are on the head end. Credit: C. G. Wilson 2024
Implications for New Antibiotics
The scientists think that rotifers could give important clues in the hunt for drugs to treat human infections caused by bacteria or fungi.
Antibiotics are becoming less effective because the disease-causing microbes have evolved to become resistant and no longer respond to treatment. The World Health Organization recently sounded the alarm, warning in a June report of the “pressing need” to develop new antibiotics to counter the threat of resistance.
“The recipes the rotifers are using look different from known genes in microbes,” said study author Reuben Nowell of the University of Stirling. “They’re just as long and complicated, but parts of the DNA code have changed. We think the recipe has been altered by a process of evolution to make new and different chemicals in the rotifers. That’s exciting because it might suggest ideas for future medicines.”
The genes the rotifers acquired from bacteria encode an unusual class of enzymes that assemble amino acids into small molecules called non-ribosomal peptides.
“The next phase of this research should involve identification of multiple non-ribosomally synthesized peptides produced by bdelloid rotifers, and establishment of the conditions upon which the synthesis of these compounds can be induced,” said study co-author Irina Arkhipova, senior scientist at the Marine Biological Laboratory.
One problem with developing new drugs is that many antibiotic chemicals made by bacteria and fungi are poisonous or have side effects in animals. Only a few can be turned into treatments that clear harmful microbes from the human body. If rotifers are already making similar chemicals in their own cells, they could lead the way to drugs that are safer to use in other animals, including people.
Understanding Rotifer Gene Acquisition
A big question is why rotifers are the only animals that borrow these useful genes from microbes at such high rates.
“We think it might be linked with another strange fact about these rotifers,” said Tim Barraclough, a study co-author from the University of Oxford. “Unlike other animals, we never see male rotifers. Rotifer mothers lay eggs that hatch into genetic copies of themselves, without needing sex or fertilization.”
According to one theory, animals that copy themselves like this can become so similar that it starts to be unhealthy. “If one catches a disease, so will the rest,” explained Barraclough. Because bdelloid rotifers don’t have sex, which allows the parental genes to recombine in beneficial ways, the rotifer mother’s genome is directly transferred to her offspring without introducing any new variation.
“If rotifers don’t find a way to change their genes, they could go extinct. This might help explain why these rotifers have borrowed so many genes from other places, especially anything that helps them cope with infections,” said Barraclough.
Nowell thinks there is much more to learn from rotifers and their stolen DNA “The rotifers were using hundreds of genes that aren’t seen in other animals. The antibiotic recipes are exciting, and some other genes even look like they’ve been taken from plants. The findings are part of a growing story about how and why genes get moved between different kinds of life,” he said.
Reference: “Bdelloid rotifers deploy horizontally acquired biosynthetic genes against a fungal pathogen” by Reuben W. Nowell, Fernando Rodriguez, Bette J. Hecox-Lea, David B. Mark Welch, Irina R. Arkhipova, Timothy G. Barraclough and Christopher G. Wilson, 18 July 2024, Nature Communications.
DOI: 10.1038/s41467-024-49919-1
Source link



